Seliwanoff’s test is a compound test which separates aldose and ketose sugars. Ketoses are differentiated from aldoses by means of their ketone/aldehyde usefulness. If the sugar contains a ketone bunch, it is a ketose. If the sugar contains an aldehyde bunch, it is an aldose. This test is much like Bial’s test. This test depends on the rule that, when heated, ketones are more quickly dried out than aldoses. It is named after Theodor Seliwanoff, the scientific expert, who performed the test. At the point when it is added to a solution containing ketones, a red shading is framed quickly giving a positive test. At the point when added to an element containing aldoses, a slower framing pink light is seen. Let’s move with seliwanoff test to distinguish sucrose from fructose.
Reagents used in the Seliwanoff Test:
Here are two things that you require to perform the experiment.
- Seliwanoff’s Reagent. (You are going to know ahead how to prepare this reagent).
- The material in which you are trying to test the presence of Keto sugar.
How to prepare Seliwanoff’s Reagent?
To prepare this reagent you will need HCL, distilled water, and resorcinol. Once you have these three things you are ready to prepare the reagent for the test.
To do this add 34ml of hydrochloric acid in 68 ml of distilled water and then after that, add 0.15 ml of resorcinol. This is how you prepare the reagent for the test and now you can perform the test. If you are thinking why HCL is needed to prepare the reagent then here is some info on HCL.
Hydrochloric Acid:
Hydrochloric acid is a solution of hydrogen chloride in the water. It is one of the most important compounds in chemistry. HCL is the chemical formula for this acid. It is used in many chemical tests. In this test, it works as an important agent. You are going to know the important role of this acid once you start reading the test ahead.
Seliwanoff’s Test Principle:
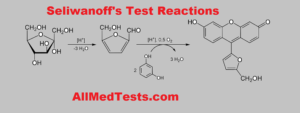
Seliwanoff Test is a test to separate keto sugars structure Aldo sugars. Keto sugars get dried out in the nearness of concentrated acids to yield furfurals or their subsidiaries which react with resorcinol in Seliwanoff reagent to yield a cherry-red hue complex as the positive result. A cherry-red shading shows the nearness of ketohexoses. Ketopentoses yield blue-green series while aldoses and disaccharides give no shading change.
With this test, fructose will bring about cherry red shading, while the aldose sugars like glucose will give a negative result with no red shading showing up in the series. In any case, if the warming is not as it is expected (which is more than 5 minutes), aldose sugar will create a pink shading. While the sucrose (a blend of fructose and glucose) will create a cherry red shading as a result of the fructose in it.
The reactions are simplified in the image below:
Seliwanoff’s Test Procedure:
Performing this test doesn’t require a complex procedure. It is just a simple qualitative test in which we differentiate aldose from ketones. To perform the test, just follow the steps I have mentioned below and you will be done within a matter of seconds. Also perform this test you require a test tube, reagent that I have already mention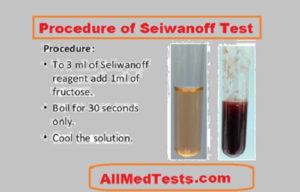 ed in a previous heading and material required. Having all these things you are good to go with the test.
ed in a previous heading and material required. Having all these things you are good to go with the test.
- To start the test, take a test tube and add 5 ml of Seliwanoff’s reagent in it. Make sure that the amount of reagent you are adding in the test tube doesn’t exceed 5 ml and measure it before pouring it.
- Now the material which is to be tested, pick it up, measure 1 ml of it and pour it down in the test tube.
- The third step should be to heat the solution using boiling water. Boil the solution in boiling water for 5 minutes and then wait on for the results. After sometime you will observe the color changes in the test tube.
Seliwanoff’s Test Precautions:
To perform every test you have got to follow some precautions. Here are some precautions to successfully perform this test.
- Before adding the reagent in the test tube, measure it carefully and make sure that it is 5 ml.
- Also, make sure that you are adding 1 ml of material to be tested because this is for the exact compatibility of the both agents.
- Make sure that what’s inside the test tube never makes a contact with any human because it is highly corrosive and can bring serious damage to the skin.
Seliwanoff’s Test Results:
After the test is being performed, you will observe the color changes in the solution. If the color turns to Red, then this means that your results are positive and keto sugar is present inside the solution.

Final Words:
If you have read this guide carefully and followed each and everything, then I am sure that your results will be 100% positive. Did you like our article on Seliwanoff’s Test? Leave us your feedback in the comments section and keep visiting our site for more medical tests.

I LOVE IT, it is very helpful. I couldn’t understand my lecturer, but I got my help here. Thank you!
It was really helpful!! Thank you!
thanks for breaking it down for my understanding.
Quite helpful. Very detailed. Thank you.
Thank you
Very helpful.
Resorcinol is solid forms but how we take it resorcinol in grams and use in liquid form
Why ketoses repond more quickly than aldoses in this test?
because of their structure.
you made it simple!! thank u
Thank you very much for help me to understand this things better than my lecture.
Thanks for help me to understand this concept
Thus is so helpful!!Thank you!
Do you know any books that i can reference regarding these kinds of medical tests? Where did you get all these knowledge?
i think these are not available in biochemistry books…
and being a medical student we perform these tests in 1st year and 2nd year MBBS in biochem lab.
Tnxx so much
it’s helpful and more understandable
Wow!Dis site is really helpful!
Very helpful article
I realy like it and its understandable but no references,bcus i nid references for dis my assignment.Thanks
will try to add references in future.
glad to know that it was helpful.
very interested explanation
Thanks a lot
Nice explanation